K-Yoddoogi Gets Permission to Manufacture Medical Devices

최고관리자
2023-07-19 15:33
45
0
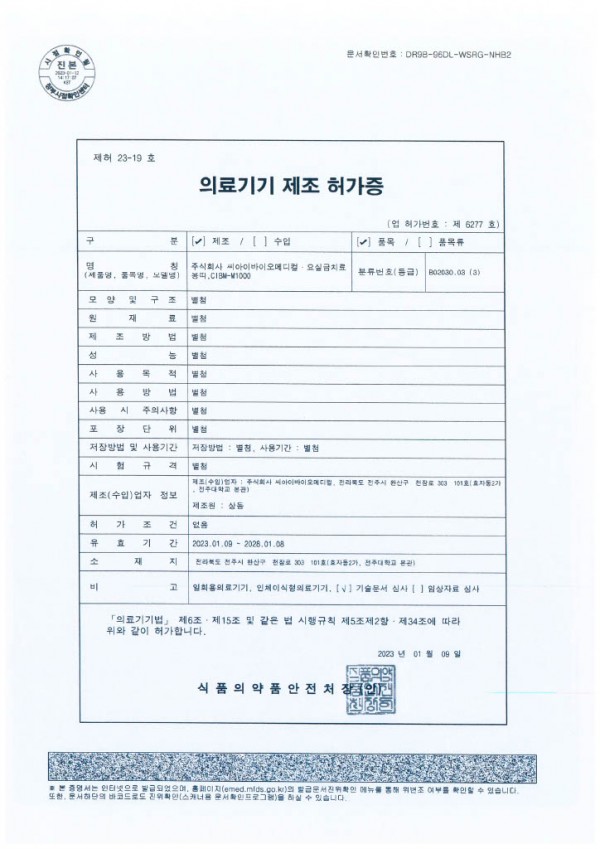
On January 9, 2023, K-Yoddoogi manufactured by CIBM received permission from the Ministry of Food and Drug Safety to manufacture medical devices.
Medical devices are directly related to human life and health, so they must have necessary facilities and manufacturing quality management systems prescribed by laws and regulations
CIBM has secured high-tech facilities and strict quality control systems, such as installing clean rooms and obtaining medical device quality management system certificates (ISO) and medical device manufacturing and quality control standards (GMP).
The CIBM K-Yoddoogi, which was approved to manufacture medical devices this time, is more sophisticated and delicate than the existing mini-sling products, so it is named "Yottoe" to mean that incontinence will stop.
K-Yoddoogi is an innovative product evaluated at home and abroad that has opened a new horizon in urinary incontinence treatment by completely solving various problems of existing products, such as automatic tension control.
There are a lot of requests for technical support not only in Korea but also in China and Mongolia for surgical methods using K-yoddoogi.
With the permission to manufacture medical devices, CIBM will continue to do its best to innovate and develop technologies so that it can become a global company.
Medical devices are directly related to human life and health, so they must have necessary facilities and manufacturing quality management systems prescribed by laws and regulations
CIBM has secured high-tech facilities and strict quality control systems, such as installing clean rooms and obtaining medical device quality management system certificates (ISO) and medical device manufacturing and quality control standards (GMP).
The CIBM K-Yoddoogi, which was approved to manufacture medical devices this time, is more sophisticated and delicate than the existing mini-sling products, so it is named "Yottoe" to mean that incontinence will stop.
K-Yoddoogi is an innovative product evaluated at home and abroad that has opened a new horizon in urinary incontinence treatment by completely solving various problems of existing products, such as automatic tension control.
There are a lot of requests for technical support not only in Korea but also in China and Mongolia for surgical methods using K-yoddoogi.
With the permission to manufacture medical devices, CIBM will continue to do its best to innovate and develop technologies so that it can become a global company.


댓글목록0
댓글 포인트 안내